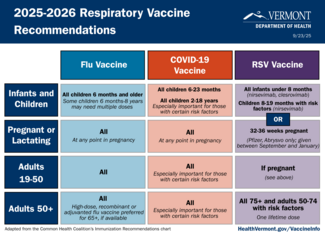
2025-2026 Respiratory Virus Season
The Health Department issued updated guidance on flu, COVID, and RSV vaccines. Read the:
- Health Advisory: Updated Respiratory Virus Vaccine Guidance (September 18, 2025)
- Standing Order for Administration of Vaccine for Pharmacists (September 17, 2025)