Automate Case Reporting
Electronic case reporting (eCR) automatically generates and transmits case reports in near real-time from electronic health records to public health agencies for review and action.
eCR Automatically Routes Reports from Providers to Public Health Agencies.
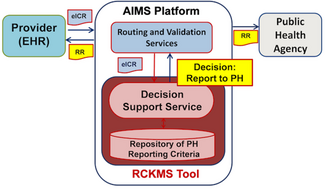
- The electronic health record system (EHR) is eCR-enabled or integrated with the eCR Now FHIR APP and has imported trigger codes.
- An eCR is triggered in the EHR and the report is sent to the AIMS platform, which is managed by the Association of Public Health Laboratories (APHL).
- The public health agency (PHA) authors reporting criteria in the Reportable Conditions Knowledge Management System (RCKMS). Each case report is assessed against reporting criteria authored by the PHA and if it is a match, AIMS sends the case report information in the form of an initial electronic case report (eICR) to the PHA. A Reportability Response (RR) is also sent to both the EHR and the PHA.
- The eICR and RR files are sent to a surveillance system, where the case will be reviewed by the PHA.
Utilizing eCR will benefit both healthcare facilities and public health staff. Automating case reporting will reduce workload burden from manual reporting, as well as improve reporting timeliness, completeness, and accuracy.
For more information, please visit: Why is eCR Important CDC
- Check that your facility is ready for eCR. Review the Readiness and Implementation Checklist.
- Review the list of EHR/Health IT products that are ready for onboarding, and make sure the product you use is on the list. Please see the ready to onboard list under For healthcare organizations. There is a list of additional pre-requisites to verify as well.
- To register your intent to send eCR, please reach out to the Health Department's eCR team by email at [email protected]. Tell us that you are interested in sending eCR, which health network you are affiliated with, and which EHR or EMR product you use.
- Once you have verified prerequisites and communicated your intent with the Health Department, please engage with the CDC’s eCR team and APHL (Association of Public Health Laboratories). They will work with your team to onboard for eCR. More information on the process can be found here: Readiness and Implementation Checklist (aimsplatform.org)
Please visit the eRSD (Electronic Reporting and Surveillance Distribution) site and register to access important triggering and reporting guidance for eCR. By registering, you will receive important notices and content updates that should be applied to your system. Implementing eRSD updates will ensure your facility is using the most up to date triggering codes and guidance.
- eRSD versions 1 and 2 will be sunset as of January 31, 2026. Please note that facilities can continue to utilize version 1 and version 2 after that date, however, there will be no updates, patches, or technical support.
- We are encouraging all facilities to adopt eRSD version 3 as soon as they are able. eRSD version 3 will provide eCR compliance, interoperability, and accurate reporting logic.
For more information on EHR triggering for eCR, please visit the AIMS site for EHR implementers.
